GREEN HYDROGEN
The golden boy of the new chemical industry
How to produce 'green' hydrogen may become a Dutch export product. So how do they (try to) do it? There are two main ways to electrolyze water. Or will the Danes steal our thunder again, as they did with our wind turbines (by using a third method)?
PROTON EXCHANGE MEMBRANE (PEM) ELECTROLYSIS
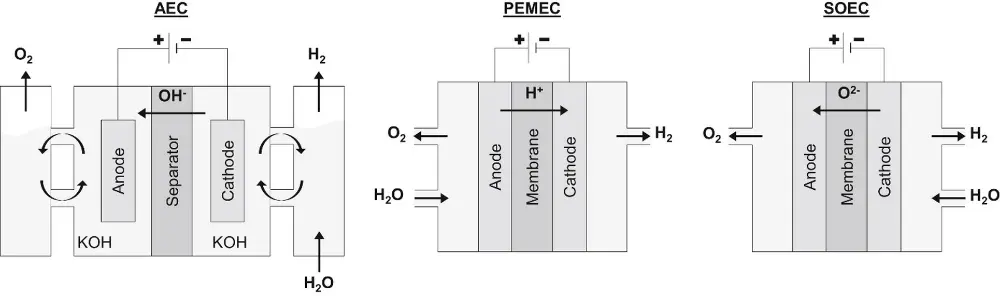
In PEM electrolysis the electrodes made of platinum or other equally expensive metals are separated by a polymer membrane, which allows hydrogen ions to pass. The membrane is usually made of perfluoro sulfonic acid (PFSA, Nafion) which has been around for more than 50 years and has been the mainstay of the chlor-alkali industry.
To test this technology on an industrial scale, Shell and ITM power are building a PEM electrolyser of 10 MW - the world's largest, so far - at Shells Rhineland refinery near Cologne. The hydrogen that is produced will be fully integrated into the refinery processes, such as the desulphurization of conventional fuels. The plant is scheduled to be in operation in 2020. The project's total investment, including integration into the refinery, is about € 20 million, with € 10 million of European funding from the European Commission's Fuel Cells and Hydrogen Joint Undertaking (FCH JU).
ALKALINE ELECTROLYSIS
Alkaline water electrolysis is cheap but has the disadvantage that it is operated at a significantly lower current density than PEM, which results in relatively bulky electrolyzers. Goal in alkaline water electrolysis is therefore to increase the current density, while retaining the good efficiency. This is possible through development of new membrane and electrode materials and improved cell designs. In alkaline water electrolysis the electrolyte typically consists of an aqueous solution of 30 wt% KOH to maximize ionic conductivity. The diaphragm between the electrodes is non-conductive to electrons, but transports hydroxide ions. The diaphragm is a thin (0.050-0.5 mm) porous foil, made of a material such as Zirfon, a composite material of zirconium oxide and Polysulfone. The electrodes can be made of Raney Nickel, which is much less expensive than platinum.
NOW WHAT ABOUT THOSE DANES?
The Danish government has awarded through its EUDP program (think SDE+) some $2.5 million towards a project called SOC4NH3, admittedly a modest sum for a modest outcome: a 50 kW electrolysis cell, nothing compared to Shell's 10 MW intentions. But it becomes interesting when you consider the technology and the use it is meant to be developed for.
Their bet is on a horse of a different color, called solid oxide electrolysis cell (SOEC).
SOLID OXIDE ELECTROLYSIS
The 'different color' comes from the ceramic membrane, which transfers oxygen ions (O2-) while it works at 900 °C, so we are dealing here with steam-electrolysis (the other ones run at 60-90 °C). The need to maintain such a high temperature might look like a disadvantage, but Haldor Topsoe, the recipient of the subsidy, found a clever way around this problem. They produce ammonia in huge amounts using nitrogen from an air separation unit (ASU) and hydrogen from a steam methane reformer, followed by a Haber Bosch (SMR-HB) plant. The high integration of these units, obtained by decades of incremental innovation, is hard to duplicate when the hydrogen comes from alkaline electrolysis. But the ASU can be eliminated when the nitrogen comes from burning some of the hydrogen made by the electrolyser. The byproduct heat can be used in the HB process and to heat the electrolysis stack. This way of integration can rival the SMR-HB process and has the potential to reduce both capex and opex. This opens the door for small ammonia plants, for which an ASU is too expensive.
The SOEC-HB is estimated to produce green ammonia with a specific energy consumption of about 26 GJ per ton, so its green ammonia plant could be more energy efficient than today's best state-of-the-art natural gas-fed ammonia plant, which consumes around 28 GJ per ton. When this gets off the ground, SOEC-HB can soak up any excess electricity that sun and wind can provide.
For a comparison of the three methods, click here
For the calculation of the levelized cost of green hydrogen production, click here
